|
One Dental Pty Ltd |

|
|---|
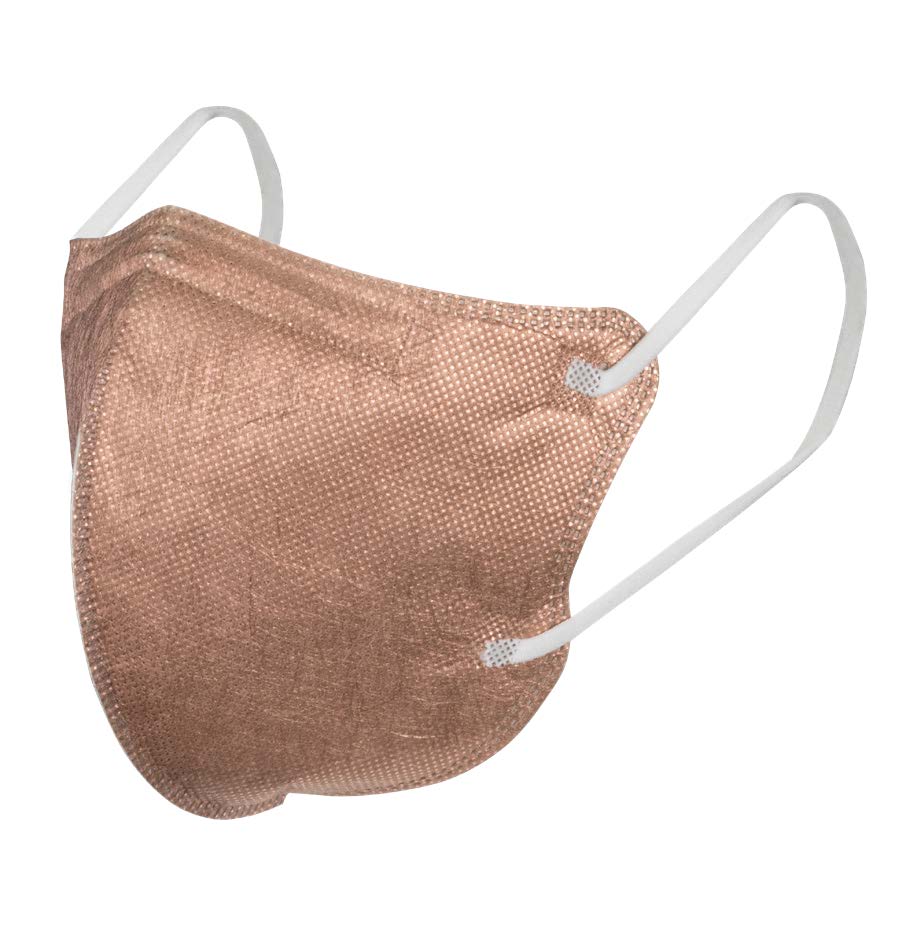
Face Mask Survivon Copatac P2/N95 Copper Butterfly respirator, Std, Box of 40
Technical Data: P2 Surgical Respirator
Surgical / Medical Respirator
General information
• High-quality particulate-filtering facepiece respirator
• Copatac™ copper coating on outer surface.
• Designed to achieve a very close and comfortable facial fit. The edges of our respirators are designed to form a seal
around the nose and mouth, whilst being easy to fit and remove.
Features
• Included in ARTG - ARTG ID: 384806
• Australian made incorporating Australian developed Copatac™ technology
• Conforms to AS/NZS1716:2012 and fluid resistance AS 4381:2015
• Particle Filtration Efficiency > 95% (tested > 99% PFE)
• Fluid resistance > 160mmHg
• Low inhalation and exhalation resistance for easy breathing
• 4 layer construction
• Ergonomically designed
• Adjustable double core wire nosepiece
Use and Applications
• This surgical respirator is intended to be worn over the nose and mouth of a person to protect users from the transfer of:
› Microorganisms
› Blood and other bodily fluids
› Airborne particulates
› Dust and pollen
Certification and Approvals
• ARTG ID: 384806
• P2 Surgical Respirator tested to AS/NZS 1716:2012
• Fluid resistance tested to ISO 22609 as per AS 4381:2015
Packaging Information
• Individually sealed
• 40 respirators in a box
Product Code: MASKP2CBF40
Category: INFECTION CONTROL
Face Mask Survivon Copatac P2/N95 Copper Butterfly respirator, Std, Box of 40Product Code: MASKP2CBF40 Category: INFECTION CONTROL Description:
Technical Data: P2 Surgical Respirator Surgical / Medical Respirator General information • High-quality particulate-filtering facepiece respirator • Copatac™ copper coating on outer surface. • Designed to achieve a very close and comfortable facial fit. The edges of our respirators are designed to form a seal around the nose and mouth, whilst being easy to fit and remove. Features • Included in ARTG - ARTG ID: 384806 • Australian made incorporating Australian developed Copatac™ technology • Conforms to AS/NZS1716:2012 and fluid resistance AS 4381:2015 • Particle Filtration Efficiency > 95% (tested > 99% PFE) • Fluid resistance > 160mmHg • Low inhalation and exhalation resistance for easy breathing • 4 layer construction • Ergonomically designed • Adjustable double core wire nosepiece Use and Applications • This surgical respirator is intended to be worn over the nose and mouth of a person to protect users from the transfer of: › Microorganisms › Blood and other bodily fluids › Airborne particulates › Dust and pollen Certification and Approvals • ARTG ID: 384806 • P2 Surgical Respirator tested to AS/NZS 1716:2012 • Fluid resistance tested to ISO 22609 as per AS 4381:2015 Packaging Information • Individually sealed • 40 respirators in a box |
 |

